Multiple Choice
Refer to the table below. 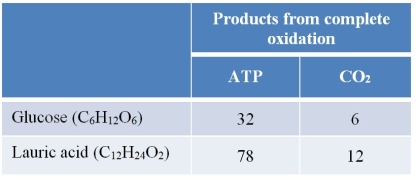 The table summarizes the products (in moles) of the complete oxidation of 1 mole of glucose and the complete oxidation of 1 mole of lauric acid, a fatty acid.Use this information to determine how energy production will compare when an equivalent amount of carbon dioxide is produced from oxidation events involving these two molecules.
The table summarizes the products (in moles) of the complete oxidation of 1 mole of glucose and the complete oxidation of 1 mole of lauric acid, a fatty acid.Use this information to determine how energy production will compare when an equivalent amount of carbon dioxide is produced from oxidation events involving these two molecules.
A) The energy production will be equal for equal quantities of CO2 production by these two molecules.
B) The energy production by lauric acid oxidation will be greater by 10 ATP when the two molecules are fully oxidized to produce equal quantities of CO2.
C) The energy production by glucose oxidation will be greater by 10 ATP when the two molecules are fully oxidized to produce equal quantities of CO2.
D) The energy production by lauric acid oxidation will be greater by 14 ATP when the two molecules are fully oxidized to produce equal quantities of CO2.
E) The energy production by glucose oxidation will be greater by 14 ATP when the two molecules are fully oxidized to produce equal quantities of CO2.
Correct Answer:

Verified
Correct Answer:
Verified
Q177: The carbon end product of glycolysis is<br>A)
Q178: Which statement about NADH is true?<br>A) NADH
Q179: In eukaryotes, the proton-motive force allows protons
Q180: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q181: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q183: Oxygen is used by<br>A) glycolysis.<br>B) the citric
Q184: During alcoholic fermentation, NAD<sup>+</sup> is regenerated from
Q185: Life in its earliest stages was very
Q186: Malate dehydrogenase is responsible for catalyzing which
Q187: Pyruvate oxidation generates<br>A) acetyl CoA and carbon