Essay
Refer to the graphs below, illustrating the free energy changes that occur during reactions in which glucose is oxidized to carbon dioxide and water. 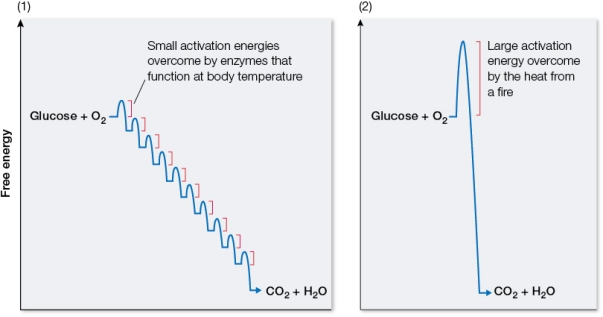 Identify which graph corresponds to the laboratory oxidation of glucose and which corresponds to cellular oxidation of glucose.Explain how the overall free energy change compares in these two reactions and why.Also compare the form that the released energy takes in these two reactions.
Identify which graph corresponds to the laboratory oxidation of glucose and which corresponds to cellular oxidation of glucose.Explain how the overall free energy change compares in these two reactions and why.Also compare the form that the released energy takes in these two reactions.
Correct Answer:

Verified
Graph 1 represents glucose oxidation in ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q188: Refer to the table showing an accounting
Q189: Atoms in triglycerides could be used in
Q190: Examine the figure below, then answer the
Q191: Oxidation and _ always occur together.
Q192: How many moles of high-energy phosphates are
Q194: Organisms regulate certain metabolic enzymes in cells
Q195: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q196: Catabolic interconversion is a metabolic pathway in
Q197: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q198: Which component of the respiratory chain is