Multiple Choice
An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.The magnitude of the orbital angular momentum of the atom is closest to:
A) 1.0 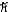
B) 1.2 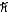
C) 1.4 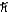
D) 1.7 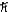
E) 2.0 
Correct Answer:

Verified
Correct Answer:
Verified
Q3: A hydrogen atom makes a downward transition
Q4: All stationary states of the hydrogen atom
Q5: Which of the statements below is true?<br>A)For
Q6: It is impossible for an electron to
Q7: Light excites atomic hydrogen from its lowest
Q9: Radio astronomers often study the radiation emitted
Q10: Do all transitions (excluding fine and hyperfine
Q11: In Bohr's model of the hydrogen atom,electrons
Q12: One of the emission lines described by
Q13: A hydrogen atom is excited to the