Multiple Choice
Part of the energy level diagram of a certain atom is shown below.The energy spacing between levels 1 and 2 is twice that between 2 and 3.If an electron makes a transition from level 3 to level 2,the radiation of wavelength λ is emitted. 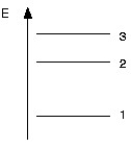 What possible radiation wavelengths might be produced by other transitions between the three energy levels?
What possible radiation wavelengths might be produced by other transitions between the three energy levels?
A) both λ/2 and λ/3
B) only λ/2
C) both 2λ and 3λ
D) only 2λ
Correct Answer:

Verified
Correct Answer:
Verified
Q56: A light beam from a 2.1 mW
Q57: Electrons with a speed of 2.1 ×
Q58: What is the velocity of an electron
Q59: Electrons are sent through a double-slit apparatus,and
Q60: A photon of blue light<br>A)has a smaller
Q61: A proton has a speed of 7.2
Q62: A beam of light having frequency greater
Q63: Gamma rays are photons with very high
Q64: When the surface of a metal is
Q66: A beam of light having frequency greater