Multiple Choice
A small piece of aluminum (atomic number 13) contains 1015 atoms.(The atomic number is the number of protons;it determines the (positive) electric charge of the nucleus and,thus,the number of electrons in a neutral atom. ) If the piece of aluminum has a net positive charge of 3.0 μC,what fraction of the electrons that the aluminum had when it was neutral would have had to be lost?
A) 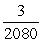
B) 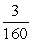
C) 1.9 × 10-20
D) 1.4 × 10-21
Correct Answer:

Verified
Correct Answer:
Verified
Q9: If the electric field strength is 900
Q10: Three charges of magnitude 3.0 <font face="symbol"></font>
Q11: A metal sphere is insulated electrically and
Q12: The charge at the bottom of the
Q13: Two like charges of the same magnitude
Q15: If the electric field strength is 500
Q16: Suppose a van de Graaff generator builds
Q17: An asteroid has acquired a net charge
Q18: One charged plastic ball is vertically above
Q19: What is the magnitude of an electric