Multiple Choice
Use the fact that the quantity of a radioactive substance after t years is given by , where is the original amount of radioactive material and k is its half-life (the number of years it takes for half the radioactive substance to decay) . 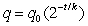
 The half-life of carbon-14 is 5600 years. Find the amount of carbon-14 remaining after 11,500 years if Round your answer to four decimal places if required.
The half-life of carbon-14 is 5600 years. Find the amount of carbon-14 remaining after 11,500 years if Round your answer to four decimal places if required. 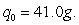
A) 29.2547 g
B) 57.4609 g
C) 0.0001 g
D) 9.8764 g
E) 20.5 g
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Simplify the expression by using the properties
Q37: Rationalize the denominator and then simplify. <img
Q38: The Richter scale reading for an earthquake
Q39: Replace the radical with a fractional exponent.
Q40: Use the properties of exponents to simplify
Q42: If $P is invested for n years
Q43: Evaluate the following expression if it represents
Q44: Use the rules of exponents to simplify
Q45: Write the expression in radical form. Do
Q46: Use the properties of exponents to determine