Multiple Choice
If we double the root-mean-square speed (thermal speed) of the molecules of a gas, then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of 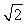 .
.
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: An ideal gas is kept in a
Q28: The number of molecules in one mole
Q44: What is the mean free path of
Q45: A sealed 89-m<sup>3</sup> tank is filled with
Q47: 2.0 L of an ideal nitrogen gas
Q50: A container is filled with a mixture
Q51: The root-mean-square speed (thermal speed) of the
Q52: The root-mean-square speed (thermal speed)of the molecules
Q53: The interior of a refrigerator has a
Q54: Assuming the radius of diatomic molecules is