Multiple Choice
The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of nitrogen is 14 g/mol. 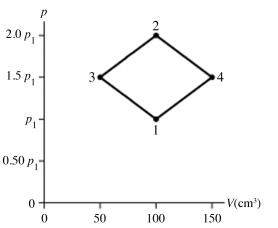
A) (a) 86 atm, (b) 700°C.
B) (a) 19 atm, (b) 700°C.
C) (a) 86 atm, (b) 160°C.
D) (a) 19 atm, (b) 160°C.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: When a fixed amount of ideal gas
Q18: An ideal gas increases in temperature from
Q19: 3.0 moles of an ideal gas with
Q26: An ideal gas is allowed to expand
Q28: When a gas undergoes an isothermal process,there
Q44: A monatomic ideal gas undergoes an isothermal
Q45: How much work is done by 3.00
Q46: An expansion process on an ideal diatomic
Q49: The gas in a perfectly insulated system
Q52: An expansion process on an ideal diatomic