Multiple Choice
The figure shows a pV diagram for 0.0066 mol of gas that undergoes the process 1 → 2 → 3. What is the pressure p2. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 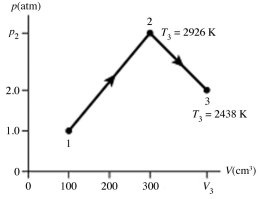
A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The figure shows a pV diagram for
Q2: A cylinder contains 24.0 moles of an
Q7: During an adiabatic process, 20 moles of
Q7: An ideal gas with γ = 1.67
Q10: A container with rigid walls is filled
Q15: When a fixed amount of ideal gas
Q32: An adiabatic compression is performed on an
Q41: A quantity of ideal gas requires 800
Q43: During an adiabatic process,an ideal gas does
Q52: A cylinder contains 23 moles of an