Multiple Choice
A measurement of an electron's speed is 2.0 × 106 m/s and has an uncertainty of 10%. What is the minimum uncertainty in its position? ( 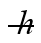 = 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
= 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
A) 0.15 nm
B) 0.29 nm
C) 0.44 nm
D) 0.60 nm
E) 0.80 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: When a certain metal is illuminated by
Q6: An 84-kW AM radio station broadcasts at
Q7: X-rays of energy 2.9 × 10<sup>4</sup> eV
Q8: In a particular case of Compton scattering,
Q9: A metal surface has a work function
Q11: A laser produces a beam of 4000-nm
Q12: A beam of x-rays at a certain
Q13: A metal having a work function of
Q14: The lifetime of an excited nuclear state
Q15: A light beam from a 2.1-mW He-Ne