Short Answer
The excited state of a certain atom is 3.2 eV ± 0.21 eV. ( 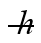 = 1.055 × 10-34 J ∙ s = 6.591 × 10-16 eV ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s = 6.591 × 10-16 eV ∙ s, 1 eV = 1.60 × 10-19 J)
(a) What is the average lifetime of this state?
(b) If the excited energy were doubled to 6.4 eV ± 0.21 eV, how would the lifetime be affected?
Correct Answer:

Verified
(a) 0.78 fs
(b) unc...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
(b) unc...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q14: The lifetime of an excited nuclear state
Q15: A light beam from a 2.1-mW He-Ne
Q16: A photon of wavelength 29 pm is
Q17: A nonrelativistic proton is confined to a
Q18: An unstable particle produced in a high-energy
Q19: If the accuracy in measuring the position
Q21: A nonrelativistic electron is confined to a
Q23: A certain particle's energy is measured by
Q24: When a metal surface is illuminated with
Q37: If the accuracy in measuring the velocity