Multiple Choice
If an electron has spin quantum number ms = - 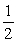 what is the possible value for the orbital quantum number l of the electron?
what is the possible value for the orbital quantum number l of the electron?
A) 0
B) 1
C) 2
D) 11
E) All of the above numbers are possible.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: An s state (l = 0) energy
Q2: An electron in a hydrogen atom has
Q3: An electron in a hydrogen atom has
Q4: What is the minimum speed needed by
Q8: In the ground state, the quantum numbers
Q9: If two electrons in an atom have
Q10: What is the correct electronic configuration for
Q11: The correct ground state electron configuration of
Q23: Consider the n = 9 shell.<br>(a)What is
Q39: An atom in a state with its