Multiple Choice
What is the greatest magnitude of the orbital angular momentum L for an electron in a state with principal quantum number n = 5?
A) 4.47 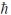
B) 4.90 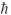
C) 5 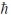
D) 5.48 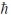
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: An atom has completely filled inner shells
Q16: The only INVALID electron state and shell
Q22: An electron in a hydrogen atom is
Q25: The only VALID electron state and shell
Q27: The normalized wave function for a hydrogen
Q29: A neutral atom has an electron configuration
Q34: An atom with 5 electrons is in
Q35: Consider the four quantum numbers of an
Q37: If the principal quantum number of an
Q44: The magnitude of the orbital angular momentum