Multiple Choice
The vibrational frequency of an HF molecule is 8.72 × 1013 Hz and the reduced mass of the molecule is 1.589 × 10-27 kg. What is the ground state vibrational energy of an HF molecule? (1 eV = 1.60 × 10-19 J, 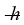 = 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
= 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
A) 0.12 eV
B) 0.18 eV
C) 0.24 eV
D) 0.30 eV
E) 0.36 eV
Correct Answer:

Verified
Correct Answer:
Verified
Q5: A diatomic molecule has 18 × 10<sup>-5</sup>
Q6: An unfilled electron state in the valence
Q6: A certain diatomic molecule emits a photon
Q7: The moment of inertia of a fluorine
Q8: A diatomic has a moment of inertia
Q9: A certain diatomic molecule emits a photon
Q10: Estimate the rotational energy (in eV) for
Q13: A p-type semiconductor has a net positive
Q22: A rotating diatomic molecule has rotational quantum
Q24: Covalent bonding is due to<br>A) the sharing