Multiple Choice
A diatomic molecule is vibrating in its first excited quantum state above the ground state. In that excited state, its frequency is 2.0 × 1013 Hz. What is the energy of the molecule in this state? (h = 6.626 × 10-34 J ∙ s, 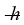 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J
A) 0.041 eV
B) 0.083 eV
C) 0.12 eV
D) 0.15 eV
E) 0.17 eV
Correct Answer:

Verified
Correct Answer:
Verified
Q3: If one metal has double the number
Q7: If a metal had a Fermi level
Q9: In a p-type semiconductor,a hole is<br>A) a
Q16: A certain molecule has 2.00 eV of
Q22: The spacing of the atoms (treated as
Q24: A diatomic molecule has 2.6 × 10<sup>-5</sup>
Q26: For a solid in which the occupation
Q28: 19) Silver has a Fermi level (energy)
Q28: Ionic bonding is due to<br>A) the sharing
Q29: The Fermi energy of rubidium at a