Multiple Choice
The stability of 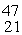 Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 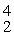
He: 4.002603 u 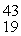
K: 42.960717 u 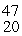
Ca: 46.954543 u 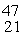
Sc 46.952409 u 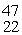
Ti: 46.951764 u
The 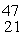
Sc nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: A radioisotope has a half-life of τ
Q32: What is the binding energy per nucleon
Q34: In a laboratory accident a work area
Q36: An archaeologist finds the <sup>14</sup>C in a
Q38: In the nuclear reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="In
Q39: The following masses are known: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg"
Q40: For the missing product X in the
Q42: The material used in certain nuclear bombs
Q47: Which of the following statements about the
Q69: A certain nucleus containing 8 protons and