Short Answer
In the nuclear reaction
n + 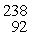
U → X + 2e-
n is a neutron and e- is an electron, and the neutrinos have not been shown. Determine the atomic mass and atomic number of the missing nuclear product X, and write X in the standard form. It is NOT necessary to identify which atom X is.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: A radioisotope has a half-life of τ
Q38: In the nuclear reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="In
Q39: The following masses are known: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg"
Q40: For the missing product X in the
Q42: The material used in certain nuclear bombs
Q44: What mass of <sup>14</sup>C (having a half-life
Q45: Calculate the reaction energy (Q value) for
Q46: For the missing product X in the
Q47: A stationary plutonium-239 nucleus decays into a
Q82: The radioactivity due to carbon-14 measured in