Multiple Choice
Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely ________. 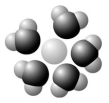
A) positively charged
B) negatively charged
C) without charge
D) hydrophobic
E) nonpolar
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: Which of the following always tends to
Q39: Consider the following reaction at equilibrium: <img
Q40: Water has a high specific heat because
Q41: Ice melts spontaneously at room temperature, even
Q43: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3735/.jpg" alt=" What results from
Q44: A solution contains 0.0000001 (10−7) moles of
Q45: During chemical evolution, which of the following
Q46: Which of the following is a property
Q47: How many electrons are involved in a
Q54: Refer to the following figure to answer