Multiple Choice
Which of these would you expect to have the lowest boiling point?
A) CH3CH2CH2OH
B) 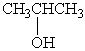
C) CH3OCH2CH3
D) CH3CH2CH2CH2OH
E) CH3CH2OCH2CH3
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Of the following compounds,the one with the
Q30: What functional group(s)is/are present in the following
Q31: Which compound would you expect to have
Q36: What group makes up the following aldehyde
Q37: What functional group(s)is/are present in the following
Q80: Ethanol,C<sub>2</sub>H<sub>5</sub>OH,and propane,C<sub>3</sub>H<sub>8</sub>,have approximately the same molar mass,yet
Q97: Draw all isomers of C<sub>6</sub>H<sub>14</sub>.
Q100: What intermolecular forces hold base pairs together
Q102: Which compound would you expect to have
Q119: Draw a structural formula for C<sub>8</sub>H<sub>18</sub>,in which