Multiple Choice
For the functional group(s) on the following molecule what characteristic IR absorption(s) would be expected (ignoring C-H absorptions) ? 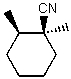
A) A peak around 1700 cm-1
B) A peak around 3300 cm-1
C) A peak around 1650 cm-1
D) A peak around 2250 cm-1
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Sodium chloride,which is quite soluble in water,is
Q13: What functional group(s)is/are present in the following
Q14: What functional group(s)is/are present in the following
Q16: What alkyl groups make up the following
Q20: An anticipated IR absorption band may not
Q21: An oxygen-containing compound shows strong IR absorption
Q22: Which molecule has a zero dipole moment?<br>A)
Q23: Which alkane is predicted to have the
Q50: The six p electrons in benzene are
Q113: Which compound would you expect to have