Multiple Choice
For the following reaction sequence (it is not necessary to understand the chemistry) what significant change(s) would be expected by IR (ignoring C-H absorptions) ? 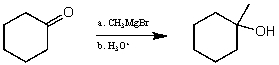
A) A peak around 1710 cm-1 would disappear and a new peak around 3300-3500 cm-1 would appear.
B) A peak around 1710 cm-1 would appear and a new peak around 1650 cm-1 would disappear.
C) A peak around 2150 cm-1 would disappear and a new peak around 3300-3500 cm-1 would appear.
D) No change would be observed.
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q103: Which molecule has a zero dipole moment?<br>A)
Q104: Which compound would have the lowest boiling
Q105: Which compound would you expect to have
Q106: The absorption band for the O-H stretch
Q107: Which molecule has a dipole moment of
Q109: Which compound is an ester? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg"
Q110: Which molecule(s)has/have dipole moment(s)equal to zero?<br>A)<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg"
Q111: Which of these is the weakest of
Q113: A tertiary carbon atom is present in
Q117: The strongest of attractive forces is which