Multiple Choice
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 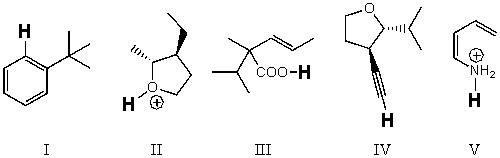
A) III > II > IV > V > I
B) II > III > V > IV > I
C) II > V > III > IV > I
D) V > III > II > IV > I
E) I > IV > II > III > V
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The four basic types of reactions are:
Q2: What does the reaction between the following
Q3: What is/are the product(s)of the following acid-base
Q4: Which base would not effectively deprotonate benzoic
Q5: Addition reactions are characteristic of compounds with
Q7: Rank the bold-faced hydrogens for the following
Q8: What is/are the product(s)of the following acid-base
Q9: What is/are the product(s)of the following acid-base
Q11: Reagents that seek to react with a
Q24: Which base would not effectively deprotonate acetylene?<br>A)LiOCH<sub>3</sub><br>B)CH<sub>3</sub>Li<br>C)CH<sub>3</sub>OCH<sub>2</sub>MgBr<br>D)KH<br>E)(CH<sub>3</sub>)<sub>2</sub>NLi