Multiple Choice
Consider the graph below,which is a plot of the relative energies of the various conformations of 2,3-dimethylbutane,viewed through the C-2-C-3 bond.The conformations corresponding to the 120o and 240o are: 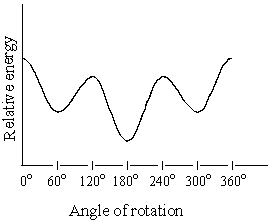
A) Eclipsed,more stable than the conformation at 0o
B) Eclipsed,more stable than the conformation at 180o
C) Staggered,more stable than the conformation at 0o
D) Staggered,less stable than the conformation at 180o
E) Two of the above are true
Correct Answer:

Verified
Correct Answer:
Verified
Q39: cis-1,3-Dibromocyclohexane is represented by structure(s): <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg"
Q40: How many moles of hydrogen (H<sub>2</sub>)will react
Q41: What is the index of hydrogen deficiency
Q42: The most stable conformation of butane is:
Q43: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg" alt=" -Which of the
Q45: The most stable conformation of 3-bromo-2-methylpentane,viewed through
Q46: What is the correct IUPAC name for
Q47: Give the IUPAC name for <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg"
Q48: The most stable conformation of cis-1-tert-butyl-2-methylcyclohexane is
Q161: What is the index of hydrogen deficiency