Multiple Choice
The graph below is a plot of the relative energies of the various conformations of: 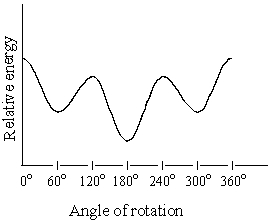
A) 2-chloropropane
B) 1,3-dichloropropane
C) 2-methylpropane
D) Butane (C1-C2 rotation)
E) Butane (C2-C3 rotation)
Correct Answer:

Verified
Correct Answer:
Verified
Q2: How many moles of hydrogen (H<sub>2</sub>)will react
Q3: Select the structure for cis-3-methyl-6-vinylcyclohexene. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg"
Q4: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg" alt=" -Which of the
Q8: What is the index of hydrogen deficiency
Q9: Which is the most stable conformation of
Q10: What is the correct name of the
Q11: How many moles of hydrogen (H<sub>2</sub>)will react
Q97: Which cycloalkane has the greatest ring strain?<br>A)Cyclopropane<br>B)Cyclobutane<br>C)Cyclopentane<br>D)Cyclohexane<br>E)Cycloheptane
Q160: What is the index of hydrogen deficiency
Q162: What is the simplest alkane,i.e.,the one with