Multiple Choice
The most stable conformation for 1,2-ethanediol (ethylene glycol) is shown below.It is the most stable conformation because: 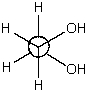
A) this corresponds to an anti conformation.
B) in general,gauche conformations possess the minimum energy.
C) it is stabilized by intramolecular hydrogen bonding.
D) it is a staggered conformation.
E) it has the highest energy of all the possibilities.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: How many moles of hydrogen (H<sub>2</sub>)will react
Q31: What is the common name for this
Q32: The IUPAC name for <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg" alt="The
Q33: What is the correct IUPAC name for
Q34: What is the index of hydrogen deficiency
Q35: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg" alt=" -Which of the
Q37: The twist boat conformation is the preferred
Q39: cis-1,3-Dibromocyclohexane is represented by structure(s): <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg"
Q40: How many moles of hydrogen (H<sub>2</sub>)will react
Q41: What is the index of hydrogen deficiency