Multiple Choice
Consider the SN2 reaction of 2-iodopentane with CH3CO2- ion. 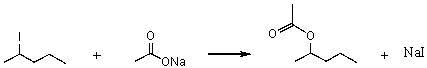 Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and CH3CO2- ion?
Assuming no other changes,what effect on the rate would result from simultaneously doubling the concentrations of both 2-iodopentane and CH3CO2- ion?
A) No effect.
B) It would double the rate.
C) It would triple the rate.
D) It would increase the rate four times.
E) It would increase the rate six times.
Correct Answer:

Verified
Correct Answer:
Verified
Q63: The product(s)for the following reaction would mainly
Q64: What final product is likely to be
Q65: Consider the S<sub>N</sub>2 reaction of 1-chloro-5-methylhexane with
Q66: The product(s)for the following reaction would mainly
Q67: What would be the major product(s)of the
Q69: Increasing the temperature of a chemical reaction
Q70: Which of the following is a
Q71: The product(s)for the following reaction would mainly
Q72: Treating 1-bromo-1-methylcyclohexane with CH<sub>3</sub>OH at room temperature
Q73: Increasing the temperature of a chemical reaction