Multiple Choice
The reaction, 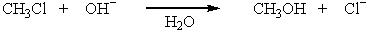 has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
A) -73.8 kJ mol-1
B) -76.8 kJ mol-1
C) -59.0 kJ mol-1
D) +91.6 kJ mol-1
E) -91.6 kJ mol-1
Correct Answer:

Verified
Correct Answer:
Verified
Q34: What would be the major product obtained
Q35: S<sub>N</sub>2 reactions of the type,Nu<sup>-</sup> +
Q37: The product(s)for the following reaction would mainly
Q38: Predict the product(s)for the following reaction sequence.
Q40: What would be the major product(s)of the
Q41: What would be the major product(s)of the
Q42: The p orbital of a methyl cation,CH<sub>3</sub><sup>+</sup>,contains
Q43: What product(s)would you expect to obtain from
Q44: What would you expect to be the
Q213: Give a detailed reaction mechanism for the