Multiple Choice
The thermodynamic parameters at 298 K for the following reaction are given below. 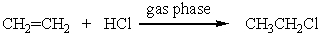 Hº = -64.9 kJ mol-1
Hº = -64.9 kJ mol-1
Sº = -131 J K-1 mol-1
Gº = -25.8 kJ mol-1
Which of the following statements is true of the reaction?
A) Both Hº and Sº favor product formation.
B) Neither Hº nor Sº favors product formation.
C) The entropy term is unfavorable but the formation of ethyl chloride is favored.
D) The entropy term is favorable but the formation of ethyl chloride is not favored.
E) The sign of Gº indicates that the reaction cannot occur as written.
Correct Answer:

Verified
Correct Answer:
Verified
Q50: Even when one or more stereogenic centers
Q61: Predict the final product(s)obtained when (2R,3R)-2-bromo-3-methylpentane is
Q63: What is the major product of the
Q64: An alkene with the molecular formula C<sub>8</sub>H<sub>16
Q66: What is the major product of the
Q67: What is the major product of the
Q68: What is the major product of the
Q69: <span class="ql-formula" data-value="\pi"><span class="katex"><span
Q117: Draw Fischer projection formula(s)of the major product(s)of
Q131: What is the chief product of the