Multiple Choice
Compound X has the molecular formula C6H10.X decolorizes bromine in carbon tetrachloride.X also shows IR absorption at about 3300 cm-1.When treated with excess hydrogen and a nickel catalyst,X yields 2-methylpentane.The most likely structure for X is:
A) 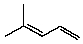
B) 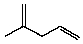
C) 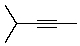
D) 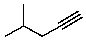
E) 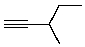
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Which reaction is regioselective? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg" alt="Which
Q11: Which alkene would react with cold dilute
Q12: Deduce the structure of an unknown compound
Q14: Which reagent or test could be used
Q16: What is the major product of the
Q17: What is the major product for the
Q18: What is the major product for the
Q19: Addition of hydrogen chloride to the following
Q20: What is the major product of the
Q161: Which of the following could be used