Multiple Choice
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C9H18O2 and characteristic 13C-NMR peaks at 11.3,21.6,25.3,49.4,67.1,and 175.5 ppm? Relative integration is shown. 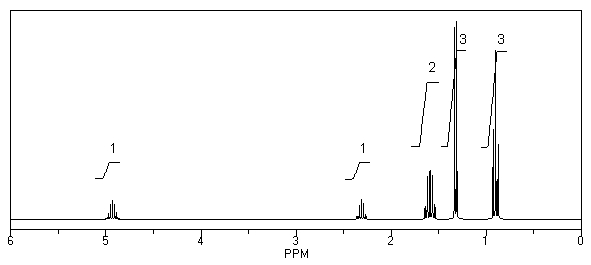
A) 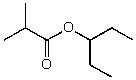
B) 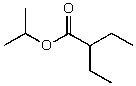
C) 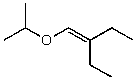
D) 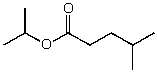
E) 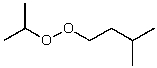
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: What is the structure of the compound
Q33: A shielded proton will absorb at a
Q34: If all the protons of 1-fluoropentane could
Q35: A downfield ( <span class="ql-formula" data-value="\delta"><span
Q37: Which one of the following best represents
Q38: The <sup>1</sup>H NMR spectrum of which of
Q39: An organic compound absorbs strongly in the
Q41: How will the methyl carbon appear in
Q96: What compound is used as the standard
Q147: Which of these compounds will not be