Multiple Choice
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C5H11NO,which shows a characteristic stretch in the IR around 1700 cm-1,and a characteristic peak at 202 ppm in the 13C-NMR? Relative integration is shown. 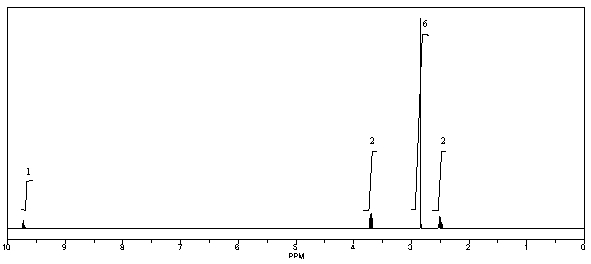
A) 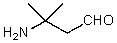
B) 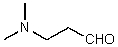
C) 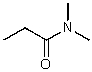
D) 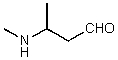
E) 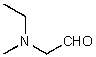
Correct Answer:

Verified
Correct Answer:
Verified
Q20: What is the structure of the compound
Q21: What is the structure of the compound
Q22: Which proton(s)of the compound below would appear
Q23: What is the structure of the compound
Q24: What is the molecular formula of
Q26: The broadband proton-decoupled <sup>13</sup>C NMR spectrum of
Q28: How many <sup>13</sup>C signals would 1,3-dichlorobenzene give?
Q29: Which proton(s)of the compound below would appear
Q30: In the structure shown,H<sub>b</sub> and H<sub>c</sub> are
Q106: Which form of electromagnetic radiation possesses the