Multiple Choice
Which of the following reactions would have the smallest energy of activation?
A) CH4 + Br· CH3· + HBr
B) CH3CH3 + Br· CH3CH2· + HBr
C) 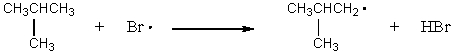
D) 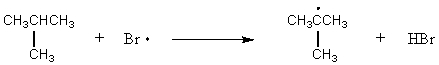
E) 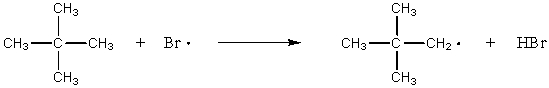
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: The free radical chlorination of pentane produces
Q28: If chlorocyclopentane were chlorinated to form all
Q34: The mechanism for a free-radical reaction consists
Q64: Draw bond-line formulas of all monochloro derivatives
Q89: An example of a reaction having
Q90: What is the product for the following
Q94: Which of the reactions listed below
Q97: What is the product for the following
Q98: What is the product for the following
Q138: The p orbital of a methyl radical