Multiple Choice
The accompanying diagram,which describes the fate of the intermediate in a reversible reaction,implies that: 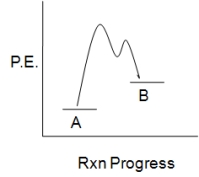
A) the less stable product forms more rapidly.
B) the more stable product forms more rapidly.
C) product B will predominate at equilibrium.
D) the intermediate has a short lifetime.
E) No conclusions can be drawn as to either reaction rate or product stability.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Which of these dienes is the most
Q7: What is (are)the product(s)of the following reaction?
Q9: Which compound would have a UV absorption
Q12: Which carbocation would be least stable? <img
Q13: Which of the following dieneophiles is most
Q92: The HOMO of the allylic radical has
Q122: The allyl radical has how many
Q172: Draw the structural formula for (2Z,4Z,6Z)-3,4,8-trimethyl-2,4,6-nonatriene,clearly indicating
Q182: Stereochemically speaking,the Diels-Alder reaction is _ and
Q195: The allyl radical has how many