Solved
A Compound Has the Molecular Formula,C6H12O2 14 And Has 20The Most Likely Structure for This Compound Is:
A)
Multiple Choice
A compound has the molecular formula,C6H12O2.Its IR spectrum shows a strong absorption band near 1740 cm-1;its 1H NMR spectrum consists of two singlets,at 1.4 and 2.0.The most likely structure for this compound is: 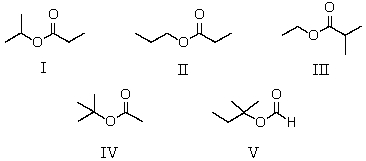
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: In which of these species are all
Q26: In which of the following sequences
Q29: Which of the following compounds is
Q41: What is the product of the
Q42: Which of the following would be the
Q47: Which of the following reactions would constitute
Q53: Which of the following acids would have
Q86: An acid chloride is prepared from the
Q150: While the IUPAC name for HCO<sub>2</sub>H is
Q169: The IR spectrum of a compound exhibits