Multiple Choice
A compound has the molecular formula C8H14O4.Its IR spectrum shows a strong absorption band near 1740 cm-1.Its 1H NMR spectrum consists of: triplet,
1.3
Singlet,
2.6
Quartet,
4.2
The most likely structure for the compound is: 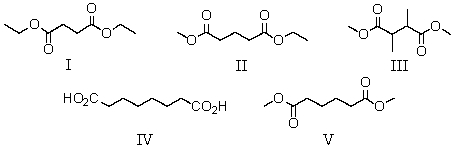
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The only carboxylic acid derivative with two
Q25: Which of the following would be the
Q109: What would be the product of the
Q112: What would be the final product? <img
Q113: Identify the product(s)of the following reaction. <img
Q115: Which of the following would yield (S)-2-butanol?<br>A)<br><img
Q117: Which compound would be most acidic?<br>A)<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg"
Q118: Which of the following combinations of reagents
Q119: Which of the following would be the
Q177: Which compound would be the weakest acid?<br>A)CHCl<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CO<sub>2</sub>H<br>B)ClCH<sub>2</sub>CHClCH<sub>2</sub>CO<sub>2</sub>H<br>C)CH<sub>3</sub>CCl<sub>2</sub>CH<sub>2</sub>CO<sub>2</sub>H<br>D)CH<sub>3</sub>CHClCHClCO<sub>2</sub>H<br>E)CH<sub>3</sub>CH<sub>2</sub>CCl<sub>2</sub>CO<sub>2</sub>H