Multiple Choice
Which reaction is likely to have more products than reactants when the reaction reaches equilibrium?
A) 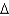 G = -25 kcal/mol
G = -25 kcal/mol
B) 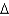 G = -50 kcal/mol
G = -50 kcal/mol
C) 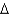 G = -75 kcal/mol
G = -75 kcal/mol
D) 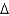 G = -100 kcal/mol
G = -100 kcal/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which of the following best illustrates the
Q12: Explain how temperature can affect enzyme activity.
Q13: Which of the following best describes why
Q18: Match each definition with the corresponding term.<br>-transition
Q30: For each situation, choose the most appropriate
Q48: Reactions that reach an equilibrium point are
Q49: Which of the following best describes how
Q53: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5641/.jpg" alt=" Photomicrograph by Dr.
Q54: Enzyme activity is increased by falling temperatures.
Q60: Which of the following is a correct