Multiple Choice
On the basis of the kinetic theory of gases,when the absolute temperature is doubled,the average kinetic energy of the gas molecules changes by a factor of
A) 16
B) 2
C) 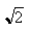
D) 4
E) 0.5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: If both the temperature and the volume
Q50: If the absolute temperature of a gas
Q51: A device used to measure the
Q52: A temperature of 14ºF is equivalent to<br>A)-10ºC<br>B)7.77ºC<br>C)25.5ºC<br>D)26.7ºC<br>E)47.7ºC
Q53: Use the following to answer the question:
Q55: Use the following to answer the question:
Q56: Boltzmann's constant,k,has a value of 1.381
Q57: The rms speed of oxygen molecules is
Q58: A temperature difference of 9ºF is the
Q59: Normal human body temperature is 98.6ºF.What is