Multiple Choice
Use the following to answer question: 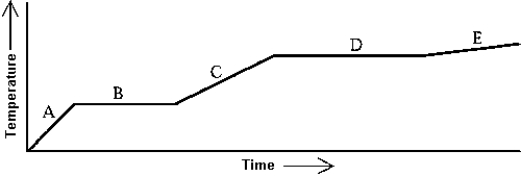
-Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the solid can be found by
A) multiplying the length of B (in seconds) by the rate at which heat is added,and dividing by the mass of the substance.
B) multiplying the length of D (in seconds) by the rate at which heat is added,and dividing by the mass of the substance.
C) dividing the rate at which heat is added by the product of the slope of A and the mass of the substance.
D) dividing the rate at which heat is added by the product of the slope of C and the mass of the substance.
E) dividing the rate at which heat is added by the product of the slope of E and the mass of the substance.
Correct Answer:

Verified
Correct Answer:
Verified
Q52: Use the following to answer the
Q53: Use the following to answer the question:
Q54: A balloon contains gas at a pressure
Q55: You add 50 g of ice cubes
Q56: When a substance goes directly from a
Q58: Use the following to answer the question:
Q59: Use the following to answer the question:
Q60: The fact that most solids have molar
Q61: From the measured molar heat capacities and
Q62: The equation of state for a certain