Multiple Choice
Use the following to answer question: 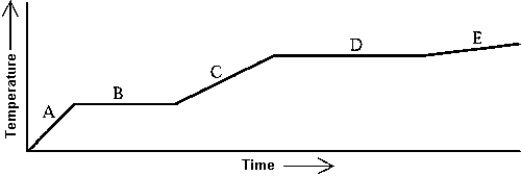
-Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.Which of the following statements is correct?
A) The latent heat of fusion is greater than the latent heat of vaporization.
B) The latent heat of vaporization is greater than the latent heat of fusion.
C) The latent heat of vaporization is equal to the latent heat of fusion.
D) The mass of the substance must be known before any statements about the latent heats can be made.
E) The relative sizes of the latent heats depend on the rate at which the heat is added.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Use the following to answer the question:
Q6: The work done by an ideal
Q7: Use the following to answer question: <img
Q8: A 1.0-kg piece of marble at 100ºC
Q9: A container contains a 200 mL
Q11: The molar heat capacity at constant volume
Q12: A gas can absorb heat without changing
Q13: On a hot summer day,water collects on
Q14: Which of the following statements about heat
Q15: A system is said to go through