Multiple Choice
Use the following to answer the question: 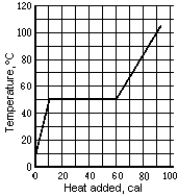
-The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the solid phase is
A) 0.6 cal/g · Cº
B) 0.25 cal/g · Cº
C) 1.6 cal/g · Cº
D) 1.7 cal/g · Cº
E) None of these is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q88: An ideal gas is heated so that
Q89: The pressure of a mass of
Q90: A small water reactor recently installed at
Q91: An ideal gas undergoes a cyclic process
Q92: Use the following to answer question: <img
Q94: The temperature of water in the
Q95: A 4-kg mass of metal of
Q96: If the heat capacities of both ice
Q97: Use the following to answer the question:
Q98: Use the following to answer the question: