Multiple Choice
Use the following to answer the question: 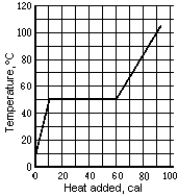
-The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the liquid phase is
A) 0.84 cal/g · Cº
B) 0.25 cal/g · Cº
C) 1.6 cal/g · Cº
D) 1.7 cal/g · Cº
E) None of these is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q63: In an adiabatic reversible compression of an
Q64: The pressure of a mass of
Q65: Some water is poured into some ice.What
Q66: In a system composed of gas contained
Q67: For most metals,the heat capacity goes
Q69: A container contains a 200 mL of
Q70: A system is said to go through
Q71: Use the following to answer the
Q72: A state variable is one that allows
Q73: In a certain thermodynamic process,20 cal of