Multiple Choice
The radii of the Bohr orbits in atomic hydrogen are given by 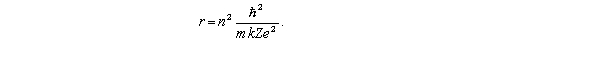 If the radius of the first Bohr orbit (n = 1) is 0.053 nm,the radius of the third Bohr orbit (n = 3) is
If the radius of the first Bohr orbit (n = 1) is 0.053 nm,the radius of the third Bohr orbit (n = 3) is
A) 0.16 nm
B) 0.018 nm
C) 0.48 nm
D) 0.35 nm
E) 1.3 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q93: The first Bohr radius,r<sub>0</sub>,is 0.0529 nm and
Q94: J.J.Thomson's model of an atom<br>A)had electrons embedded
Q95: What is the energy difference between the
Q96: The binding energy of a hydrogen atom
Q97: Bohr's quantum condition on electron orbits
Q99: Which of the following transitions are allowed
Q100: A hydrogen atom is in the state
Q101: In the Bohr model of the
Q102: An electron in a hydrogen atom jumps
Q103: Use the following figure for the next