The Equation Derived by Bohr for the Wavelengths Of Of the Lines in Hydrogen- Like Spectra Is the Of the Lines
Multiple Choice
The equation derived by Bohr for the wavelengths of of the lines in hydrogen- like spectra is 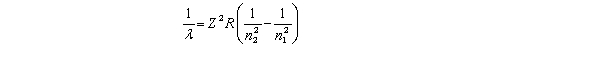 The first member of the Balmer series of hydrogen has = 660 nm.Doubly ionized
The first member of the Balmer series of hydrogen has = 660 nm.Doubly ionized 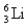 is hydrogen-like.The wavelength of the first member of the Balmer series for doubly ionized
is hydrogen-like.The wavelength of the first member of the Balmer series for doubly ionized 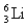 is
is
A) 73 nm
B) 5.9 103 nm
C) 150 nm
D) 60 nm
E) 1.8 10-3 nm
Correct Answer:

Verified
Correct Answer:
Verified
Q99: Which of the following transitions are allowed
Q100: A hydrogen atom is in the state
Q101: In the Bohr model of the
Q102: An electron in a hydrogen atom jumps
Q103: Use the following figure for the next
Q105: The number of oxygen's eight electrons that
Q106: What is the difference in wavelength between
Q107: A photon of wavelength 80 nm is
Q108: Z for the element whose electronic configuration
Q109: The energy of the nth level in