Multiple Choice
According to the Bohr theory,the allowed energy states for the hydrogen atom are given by the relation 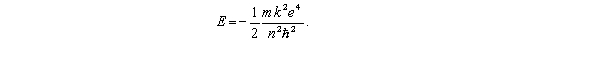 This formula can be readily extended to other hydrogenic (one-electron) systems.The energy of the second level (n = 2) for the doubly ionized lithium atom is
This formula can be readily extended to other hydrogenic (one-electron) systems.The energy of the second level (n = 2) for the doubly ionized lithium atom is
A) -54.4 eV
B) 13.6 eV
C) -30.6 eV
D) -3.4 eV
E) -1.5 eV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q36: The wavelength of the visible line in
Q37: What is the magnitude of the change
Q38: The energy of the nth level
Q39: If you measure the angular momentum of
Q40: The radius of the n = 1
Q42: The d state of an electronic configuration
Q43: The number of electrons in the M
Q44: For the hydrogen atom in the ground
Q45: Evidence of electron capture by a nucleus
Q46: The symbol that represents the orbital quantum