Multiple Choice
For the hydrogen atom in the ground state,the radial probability density is 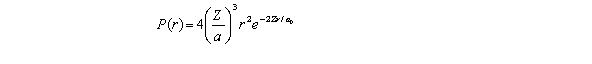 The probability of finding the electron in the range r = 0.04a0 at r = a0 is
The probability of finding the electron in the range r = 0.04a0 at r = a0 is
A) 0.0423
B) 0.0164
C) 0.0217
D) 0.0137
E) 0.0241
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: The wavelength of the K<sub> <span
Q51: The Pauli exclusion principle states that
Q52: What is the energy difference between the
Q53: The wavelength of the photon emitted when
Q54: When a gold foil is bombarded with
Q56: The electron configuration of nitrogen (Z =
Q57: When the voltage across an X-ray tube
Q58: The number of electrons in the L
Q59: The symbol that represents the principal quantum
Q60: The wavelength of the visible line in