Multiple Choice
For the hydrogen atom in the ground state,the radial probability density is 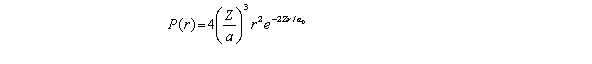 The probability of finding the electron in the range r = 0.04a0 at r = 2a0 is
The probability of finding the electron in the range r = 0.04a0 at r = 2a0 is
A) 0.0463
B) 0.0184
C) 0.0217
D) 0.0117
E) 0.0341
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: The constant in the Rydberg-Ritz formula is
Q14: It was _ who showed that the
Q15: Which of the following atomic numbers (which
Q16: If the angular momentum is characterized by
Q17: The total angular momentum is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6079/.jpg"
Q19: Which of the following statements is
Q20: In the Bohr Model of the hydrogen
Q21: For the hydrogen atom in the
Q22: The energy of a photon of visible
Q23: The order-of-magnitude of the diameter of an