Multiple Choice
An electron has a wave function given by 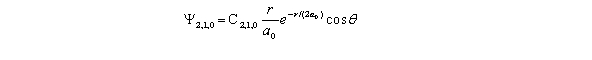 The probability of finding the electron when = 90 and = 0.5 ,and from r = a0 to 1.06a0 is approximately
The probability of finding the electron when = 90 and = 0.5 ,and from r = a0 to 1.06a0 is approximately
A) zero
B) 2.12%
C) 4.75%
D) 5.8%
E) 6.34%
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q59: The symbol that represents the principal quantum
Q60: The wavelength of the visible line in
Q61: X rays are emitted when the
Q62: According to Bohr's model,the radius of an
Q63: A hydrogen atom is in an excited
Q65: According to the Bohr theory,a hydrogen atom<br>A)does
Q66: The first Bohr radius,r<sub>0</sub>,is 0.0529 nm and
Q67: The order-of-magnitude of the diameter of the
Q68: For the hydrogen atom in the ground
Q69: What is the wavelength of the most