Multiple Choice
A hydrogen atom is in the state n = 4, 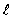 = 3.The two spectral states due to spin-orbit coupling are
= 3.The two spectral states due to spin-orbit coupling are
A) 3d7/2 and 3d5/2
B) 4f7/2 and 4f5/2
C) 4f5/2 and 4f3/2
D) 3d5/2 and 4d3/2
E) 4d7/2 and 4d5/2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Which of the following statements is
Q20: In the Bohr Model of the hydrogen
Q21: For the hydrogen atom in the
Q22: The energy of a photon of visible
Q23: The order-of-magnitude of the diameter of an
Q25: Which of the following atomic numbers (which
Q26: The p state of an electronic configuration
Q27: What is the difference in energy
Q28: Which of the following characteristic X-ray
Q29: For the principal quantum number n =