Multiple Choice
Silicon is one of the Group IV semiconductors and has an energy gap of 1.12 eV. Generally only metals have highly shiny surface, yet as shown in the figure, silicon appears to reflect like a metal. This is because 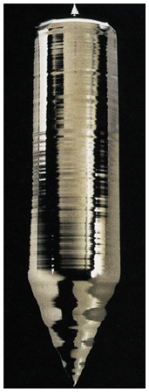
A) silicon is a good semiconductor with a well defined energy gap.
B) visible light has energies in the range 2-3 eV which is much larger than the energy gap allowing electrons to be promoted from the valence band to the conduction band.
C) the surface of silicon is smooth making it reflect like a metal.
D) silicon can be grown in very high purity. It is the impurities that make a semiconductor dull looking.
E) None of these is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: The classical theory of conduction<br>A)fails because electrons
Q35: If the energy levels of a Fermi
Q39: A metal is a good conductor because
Q43: Use the following to answer the next
Q44: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6081/.jpg" alt=" The symbol for
Q45: If the atoms are treated as solid
Q49: The free-electron model of metals gives a
Q53: Use the following to answer the next
Q67: Use the following to answer the
Q75: Copper has a mass density of