Multiple Choice
The intensity of gamma rays passing through a material as a function of thickness, x is given by 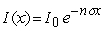 where n is the number of atoms per cm3 and is the absorption cross section. If the intensity of gamma rays of a particular energy is reduced by half when they passed through 1 cm of lead, what is the absorption cross section for the gamma rays? The density of lead is 11.3 g/cm3 and its molar mass is 207 g.
where n is the number of atoms per cm3 and is the absorption cross section. If the intensity of gamma rays of a particular energy is reduced by half when they passed through 1 cm of lead, what is the absorption cross section for the gamma rays? The density of lead is 11.3 g/cm3 and its molar mass is 207 g.
A) 3.5 * 10-

22 cm2
B) 6.8 * 10 
22 cm2
C) 4.6 * 10 
23 cm2
D) 2.1 * 10 
23 cm2
E) 8.4 * 10 
24 cm2
Correct Answer:

Verified
Correct Answer:
Verified
Q17: What are the numbers of protons Z
Q18: The nuclear radius of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6081/.jpg" alt="The
Q21: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6081/.jpg" alt=" The Q of
Q25: A particle is incident on a nucleus.The
Q34: Why is the ratio of neutrons to
Q42: For most nuclei,the total binding energy is
Q67: Neutrons are effective particles for penetrating the
Q85: The fact that most <span
Q87: A radioactive source has a half-life
Q95: A certain radioactive element has a half-life